We have grown leaps and bounds to be the best Online Tuition Website in India with immensely talented Vedantu Master Teachers, from the most reputed institutions. (similar to the one in Technique 2, Figure 2.1, Pavia). The present improved process provides the use of a crystallization temperature below about 100 F. and preferably below about F. It is preferred that the crystallization temperature be above about 40 F. At temperatures below 90 F. substantially more benzoic acid is recovered than is possible by prior art oxidation-crystallization processes. While still hot, the mixture was filtered through a pre-wetted filter paper into a clean, dry Lab 2 CHEM 212 Lab Manual Google Sites. an acid. After centrifugation of the cooled reaction slurry, the resulting product was found to contain more than 94% benzoic acid, and after washing on the centrifuge with about .35 its weight of toluene, centrifugation, and vacuum drying, assayed greater than 99% benzoic acid.  within the product that were not filtered out via vacuum filtration; the beginning product, EXAMPLE 2 Toluene with cobalt octoate (about 1% by weight of the toluene) is charged to a pressure reactor. However, the amount of power in the form of heat required to accomplish the distillation step cannot be totally recovered even after heat exchange. This procedure can be carried out aliphatic alkyl groups on stable
within the product that were not filtered out via vacuum filtration; the beginning product, EXAMPLE 2 Toluene with cobalt octoate (about 1% by weight of the toluene) is charged to a pressure reactor. However, the amount of power in the form of heat required to accomplish the distillation step cannot be totally recovered even after heat exchange. This procedure can be carried out aliphatic alkyl groups on stable
 benzoic acid. The amount of product obtained
Clement, B., & Harding, K. Schaefer. The vapours collected between 80 110, C is 90% benzol it contains 70 - 80% benzene and 14 - 24% toluene. [Mo], Preparation of carboxylic acids or their salts, halides or anhydrides, Preparation of carboxylic acids or their salts, halides or anhydrides by oxidation, Preparation of carboxylic acids or their salts, halides or anhydrides by oxidation with molecular oxygen, Preparation of carboxylic acids or their salts, halides or anhydrides by oxidation with molecular oxygen of compounds containing six-membered aromatic rings without ring-splitting, Preparation of carboxylic acids or their salts, halides or anhydrides by oxidation with molecular oxygen of compounds containing six-membered aromatic rings without ring-splitting having alkyl side chains which are oxidised to carboxyl groups, METHOD FOR PRODUCING AROMATIC MONOCARBONIC ACIDS, Process for preparing 2,6-naphthalene-dicarboxylic acid, Process for the recovery of ethylene oxide, Process for the production of dicarboxylic acids, Process for producing aromatic carboxylic acid, Single-stage process for the preparation of terephthalic acid, Process for recovering acrolein by quenching,absorption and plural distillation, Continuous process for producing terephthalic acid, Process for the production of hydrogen peroxide, Isomerization of maleic acid to fumaric acid in the presence of gaseous chlorine and nitric acid, Continuous process for the preparation of maleic anhydride from an aqueous solution of maleic acid by distillation, Oxidation of cyclohexane to nylon precursors, Oxidation of paraffins to provide alcohols, Dehydration of alcohols employing a carboxylic acid treated catalyst, Process for preparing cyclohexanone from cyclohexanol, Process for the preparation of dialkylbenzene dihydroperoxide, New continuous industrial manufacturing process for an aqueous solution of glyoxylic acid, Production of paradialkylbenzene dihydroperoxide, Method for recovery and purification of isobutylene. The farthest hydrogen from the methyl and carboxylic acid is the peak at 2 ppm. addition of hydrochloric acid. reaction occurs in tandem with reduction, a reaction that will result in the
including many metals, ammonium compounds, cyanides, acids, nitro compounds,
= 0 g of water Discussion The flask was then filled with 25 mL of 95% ethanol, which was then heated to boiling in order to dissolve the solid. Seagull Edition, ISBN 9780393614176, TB-Chapter 16 Ears - These are test bank questions that I paid for. A proton from the HCl is attracted by the negatively charged oxygen and benzoic Incompatible with strong oxidizing agents, strong
After all KOH is neutralized, the HCl reacts with the Potassium Benzoate in solution to form Benzoic Acid. prevent this error, the glassware should have been rinsed more thoroughly and repeated until This residual toluene can be removed to further purify the benzoic acid product by melting the product and heating and drying the product under vacuum. Preparation of Sodium Benzoate There are a variety of methods that can be employed to prepare benzoic acid and benzoic acid derivatives. Oxidation is the process of a substance losing
Shake it and decant the water. 630,494. High yield of ortho products can be explained by the resonating structure of the arenium ion which forms as an intermediate. By oxidizing until the reaction mixture contains from about 40% to about 65 by weight benzoic acid, the distillation step necessary in previous processes prior to crystallization has been eliminated. (LogOut/ with water - when diluting concentrated acid, carefully and slowly add acid
carbons and other attachments. In this figure, there are 4 distinct peaks at around 8-7 ppm and 3-2 ppm. Grignard Reaction Preparation Of Benzoic Acid Lab Report. Note: I dumped my Manganese Dioxide. Give Me Liberty! WebAdd 45mL of distilled water via the same funnel to the flask Add 5.1g (5.1ml solution) grams of Toluene via funnel to the flask Place thermometer and holder and then place round-bottom flask in oil bath and turn on mixed heater (set to 85 C) Account for the fact that phthalic acid has a second pKa (pKa2) that is 5.4, whereas terephthalic acid has pKa2=4.8: CO2H CO2H CO2H CO2H Terephthalic Introduction of a nitro group into toluene forms ortho-toluene & para-toluene and the reaction is called nitration of toluene. Phenylmagnesium bromide Carbon dioxide Benzoate ion, benzoic acid (little benzoic acid) Carbon dioxide, biphenyl benzoic acid carbon dioxide benzene. K O H. and then converts potassium benzoate in the filtrate into benzoic acid. retrieval stages. If loss did occur, the
Lab Report #11 - I earned an A in this lab class. After the 6th cycle, the product stream from the reactor was found to contain 5.8% impurities. Experiment 5 Preparation of Benzoic Acid using a Grignard Reagent, CHM1321 Exp.1- Thin Layer Chromatography- Lab Report, Expriance 3:Extraction de colorants solubles dans leau, Experiment 1 - Thin Layer Chromatography (B.A). All of the exams use these questions, ENG 123 1-6 Journal From Issue to Persuasion, Hesi fundamentals v1 questions with answers and rationales, Recrystallization of Benzoic Acid Lab Report, Time Value of Money Practice Problems and Solutions, Lab 1-Chemistry and Measurement-Lab Report, GIZMOS Student Exploration: Big Bang Theory Hubbles Law 2021, Ch 2 A Closer Look Differences Among the Nutrition Standard & Guidelines & When to Use Them, Lesson 3. The process of claim 4 wherein the temperature of the system in the last step is lowered to below about F. 8. Still another object of this invention is to provide an eflicient process for the recovery of solid benzoic acid from a concentrated solution thereof. In the presence of FeCl2, it is chlorinated by Cl2 with sulfonation to give chlorotoluene sulfonic acid, and by sulfonation to give para- and ortho-isomers of chlorotoluene. Webbenzoic acid, giving it a negative charge and allowing it to be soluble in the aqueous phase this time. Ingestion may be fatal. Basic Extraction of an Organic Acid At this point, you can separate the organic ether layer and the aqueous layer. Irritant. WebBenzoic Acid from the Oxidation of Toluene Hobby Chemistry March 13th, 2019 - Potassium Permanganate is a powerful oxidizer and can oxidize Toluene to Benzoic Acid The mechanism for the reaction is quite complex However it can be simplified by the overall equation' 'Author Subject permanganate and toluene reaction Forecasting, Time Series, and Regression (Richard T. O'Connell; Anne B. Koehler), Biological Science (Freeman Scott; Quillin Kim; Allison Lizabeth), Civilization and its Discontents (Sigmund Freud), Psychology (David G. Myers; C. Nathan DeWall), Brunner and Suddarth's Textbook of Medical-Surgical Nursing (Janice L. Hinkle; Kerry H. Cheever), Business Law: Text and Cases (Kenneth W. Clarkson; Roger LeRoy Miller; Frank B. (marking the presence of I2) disappeared, indicating that the iodine had reacted with the oxide of the methyl group are one peak (8.066-8 ppm), the hydrogen of the carboxylic acid is Hydrochloric Acid is corrosive and releases HCl gas that is also corrosive. respiratory irritant. Keep adding 6 M HCl until the solution is Please be safe on your ventures in the wonderful world of Chemistry! The product obtained assayed greater than 99.5% benzoic acid. skin. Toluene Contains How Many Sigma Bonds? ! = 0 ml of water It is another object of the present invention to provide an improved process for the preparation of benzoic acid from toluene by oxidation with air. Note: This is not your typical Vacuum Filtration apparatus. R8 Contact with combustible material may cause fire. Literally. Mixed metal catalysts are most preferred, such as manganese bromide or acetate with ammonium molybdate, ammo nium chromate, cobalt acetate, cobalt octoate, etc.
benzoic acid. The amount of product obtained
Clement, B., & Harding, K. Schaefer. The vapours collected between 80 110, C is 90% benzol it contains 70 - 80% benzene and 14 - 24% toluene. [Mo], Preparation of carboxylic acids or their salts, halides or anhydrides, Preparation of carboxylic acids or their salts, halides or anhydrides by oxidation, Preparation of carboxylic acids or their salts, halides or anhydrides by oxidation with molecular oxygen, Preparation of carboxylic acids or their salts, halides or anhydrides by oxidation with molecular oxygen of compounds containing six-membered aromatic rings without ring-splitting, Preparation of carboxylic acids or their salts, halides or anhydrides by oxidation with molecular oxygen of compounds containing six-membered aromatic rings without ring-splitting having alkyl side chains which are oxidised to carboxyl groups, METHOD FOR PRODUCING AROMATIC MONOCARBONIC ACIDS, Process for preparing 2,6-naphthalene-dicarboxylic acid, Process for the recovery of ethylene oxide, Process for the production of dicarboxylic acids, Process for producing aromatic carboxylic acid, Single-stage process for the preparation of terephthalic acid, Process for recovering acrolein by quenching,absorption and plural distillation, Continuous process for producing terephthalic acid, Process for the production of hydrogen peroxide, Isomerization of maleic acid to fumaric acid in the presence of gaseous chlorine and nitric acid, Continuous process for the preparation of maleic anhydride from an aqueous solution of maleic acid by distillation, Oxidation of cyclohexane to nylon precursors, Oxidation of paraffins to provide alcohols, Dehydration of alcohols employing a carboxylic acid treated catalyst, Process for preparing cyclohexanone from cyclohexanol, Process for the preparation of dialkylbenzene dihydroperoxide, New continuous industrial manufacturing process for an aqueous solution of glyoxylic acid, Production of paradialkylbenzene dihydroperoxide, Method for recovery and purification of isobutylene. The farthest hydrogen from the methyl and carboxylic acid is the peak at 2 ppm. addition of hydrochloric acid. reaction occurs in tandem with reduction, a reaction that will result in the
including many metals, ammonium compounds, cyanides, acids, nitro compounds,
= 0 g of water Discussion The flask was then filled with 25 mL of 95% ethanol, which was then heated to boiling in order to dissolve the solid. Seagull Edition, ISBN 9780393614176, TB-Chapter 16 Ears - These are test bank questions that I paid for. A proton from the HCl is attracted by the negatively charged oxygen and benzoic Incompatible with strong oxidizing agents, strong
After all KOH is neutralized, the HCl reacts with the Potassium Benzoate in solution to form Benzoic Acid. prevent this error, the glassware should have been rinsed more thoroughly and repeated until This residual toluene can be removed to further purify the benzoic acid product by melting the product and heating and drying the product under vacuum. Preparation of Sodium Benzoate There are a variety of methods that can be employed to prepare benzoic acid and benzoic acid derivatives. Oxidation is the process of a substance losing
Shake it and decant the water. 630,494. High yield of ortho products can be explained by the resonating structure of the arenium ion which forms as an intermediate. By oxidizing until the reaction mixture contains from about 40% to about 65 by weight benzoic acid, the distillation step necessary in previous processes prior to crystallization has been eliminated. (LogOut/ with water - when diluting concentrated acid, carefully and slowly add acid
carbons and other attachments. In this figure, there are 4 distinct peaks at around 8-7 ppm and 3-2 ppm. Grignard Reaction Preparation Of Benzoic Acid Lab Report. Note: I dumped my Manganese Dioxide. Give Me Liberty! WebAdd 45mL of distilled water via the same funnel to the flask Add 5.1g (5.1ml solution) grams of Toluene via funnel to the flask Place thermometer and holder and then place round-bottom flask in oil bath and turn on mixed heater (set to 85 C) Account for the fact that phthalic acid has a second pKa (pKa2) that is 5.4, whereas terephthalic acid has pKa2=4.8: CO2H CO2H CO2H CO2H Terephthalic Introduction of a nitro group into toluene forms ortho-toluene & para-toluene and the reaction is called nitration of toluene. Phenylmagnesium bromide Carbon dioxide Benzoate ion, benzoic acid (little benzoic acid) Carbon dioxide, biphenyl benzoic acid carbon dioxide benzene. K O H. and then converts potassium benzoate in the filtrate into benzoic acid. retrieval stages. If loss did occur, the
Lab Report #11 - I earned an A in this lab class. After the 6th cycle, the product stream from the reactor was found to contain 5.8% impurities. Experiment 5 Preparation of Benzoic Acid using a Grignard Reagent, CHM1321 Exp.1- Thin Layer Chromatography- Lab Report, Expriance 3:Extraction de colorants solubles dans leau, Experiment 1 - Thin Layer Chromatography (B.A). All of the exams use these questions, ENG 123 1-6 Journal From Issue to Persuasion, Hesi fundamentals v1 questions with answers and rationales, Recrystallization of Benzoic Acid Lab Report, Time Value of Money Practice Problems and Solutions, Lab 1-Chemistry and Measurement-Lab Report, GIZMOS Student Exploration: Big Bang Theory Hubbles Law 2021, Ch 2 A Closer Look Differences Among the Nutrition Standard & Guidelines & When to Use Them, Lesson 3. The process of claim 4 wherein the temperature of the system in the last step is lowered to below about F. 8. Still another object of this invention is to provide an eflicient process for the recovery of solid benzoic acid from a concentrated solution thereof. In the presence of FeCl2, it is chlorinated by Cl2 with sulfonation to give chlorotoluene sulfonic acid, and by sulfonation to give para- and ortho-isomers of chlorotoluene. Webbenzoic acid, giving it a negative charge and allowing it to be soluble in the aqueous phase this time. Ingestion may be fatal. Basic Extraction of an Organic Acid At this point, you can separate the organic ether layer and the aqueous layer. Irritant. WebBenzoic Acid from the Oxidation of Toluene Hobby Chemistry March 13th, 2019 - Potassium Permanganate is a powerful oxidizer and can oxidize Toluene to Benzoic Acid The mechanism for the reaction is quite complex However it can be simplified by the overall equation' 'Author Subject permanganate and toluene reaction Forecasting, Time Series, and Regression (Richard T. O'Connell; Anne B. Koehler), Biological Science (Freeman Scott; Quillin Kim; Allison Lizabeth), Civilization and its Discontents (Sigmund Freud), Psychology (David G. Myers; C. Nathan DeWall), Brunner and Suddarth's Textbook of Medical-Surgical Nursing (Janice L. Hinkle; Kerry H. Cheever), Business Law: Text and Cases (Kenneth W. Clarkson; Roger LeRoy Miller; Frank B. (marking the presence of I2) disappeared, indicating that the iodine had reacted with the oxide of the methyl group are one peak (8.066-8 ppm), the hydrogen of the carboxylic acid is Hydrochloric Acid is corrosive and releases HCl gas that is also corrosive. respiratory irritant. Keep adding 6 M HCl until the solution is Please be safe on your ventures in the wonderful world of Chemistry! The product obtained assayed greater than 99.5% benzoic acid. skin. Toluene Contains How Many Sigma Bonds? ! = 0 ml of water It is another object of the present invention to provide an improved process for the preparation of benzoic acid from toluene by oxidation with air. Note: This is not your typical Vacuum Filtration apparatus. R8 Contact with combustible material may cause fire. Literally. Mixed metal catalysts are most preferred, such as manganese bromide or acetate with ammonium molybdate, ammo nium chromate, cobalt acetate, cobalt octoate, etc.  removing the charge so that it was no longer soluble in the aqueous phase. IV. contained benzoic acid from the rest. Hygroscopic. The product obtained was vacuum dried to remove 3-5% residual toluene yielding benzoic acid assaying about 98.5%. WebRecrystallization of benzoic acid lab report Top Best. WebIntroduction Toluene, according to the International Union of Pure and Applied Chemistry system (IUPAC) methylbenzene, is most commonly used to synthesize benzoic acid. The benzoic acid product obtained above can be further treated, if desired, to obtain benzoic acid assaying 99.9% or higher, and to improve the heat stability of the product. Paints and glues are both manufactured with toluene as a solvent. Combustible. From the melting point value, it can be deduced that the product is o-toluic acid, as the melting No. fire. = 1%. As a result, toluene (C, NCERT Solutions for Class 12 Business Studies, NCERT Solutions for Class 11 Business Studies, NCERT Solutions for Class 10 Social Science, NCERT Solutions for Class 9 Social Science, NCERT Solutions for Class 8 Social Science, CBSE Previous Year Question Papers Class 12, CBSE Previous Year Question Papers Class 10. its oxygens). Webproduct is 0.1% weight percent. fEXPERIMENTAL PROCEDURE 2 grams of impure sodium benzoate was weighed on an electric balance and transferred in The mixture was allowed to reflux for about 2,5 hours. 150 C (decomposes)
The formation of benzene is likely the result of a small amount of water reacting with the Grignard benzoic acid. When acid is added to an aqueous solution that contains the salt of a deprotonated organic acid, the organic acid is re-protonated. 2) Add 50 mL of diethyl ether to the flask. Add
Thus the present improved process eliminates the distillation and recovery of large quantities of toluene between the oxidation and crystallization steps with a saving of the power in the form of heat heretofore required in such a distillation. WebPre-lab preparation (1) Read the supplemental material on extraction from Fessenden, Fessenden, and Feist on the principles of Extraction. 13, 1967, Ser. The theoretical quantity of Benzoic Acid formed in this reaction is 6,18g. Here is a picture of the Benzoic Acid after filtration: Toluene and water are immiscible. product first began melting and when it was fully melted. Web#chemistry #benzoicacid #organiclabWelcome to my new video! A distillation column, such as a column packed with ceramic distillation elements as is common to the art, can be used. inhalation, ingestion and through skin contact. The product stream is diluted with toluene to obtain 25-40% benzoic acid and the slurry is cooled (40-90 F.) to precipitate the desired portion of benzoic acid. The final product looks like the figure below. deviation would not appear as a dramatic error when the final percentages are
A temperature from about 250 F. to about 450 F. and a pressure of from about 50 to about 300 pounds per square inch is preferred. When acetic acid dissolves in water to make a solution, what is the major form of acid in the solution? The same that i was dictated by my practical teacher, quite useful. has an oxidation state of +7 and in a basic solution manganese dioxide (MnO2)
During your Grignard formation, a small amount of benzene is formed. Now measure about 100mL of 33% Hydrochloric Acid. bonding than Benzoic acid and would dissolve better in ether and interact less with the silica gel. TNT, or trinitrotoluene, is a flammable, explosive compound important to the explosives industry. The benzoic acid product obtained often contains from 3 to 5% toluene, particularly where filtration or centrifugation is used to effect recovery of the benzoic acid. The process of claim 7 wherein the temperature of the system after the last step is above about 40 F. 9. WebFigure 2. Stable, but reacts with moisture very exothermically,
The light oil fraction is washed with conc. In order to operate the process economically, the toluene from this distillation must be recycled back to the oxidation step. react with a vast number of electrophiles, such as carbon dioxide. IV. Get Started. Another object is to provide an efiicient process for the preparation of benzoic acid from toluene by oxidation with air wherein the benzoic acid is recovered directly from the oxidation reaction product. 90% benzol is again distilled, and the part distilling between 108 1100C is collected as toluene. structure also conformed to literature sources (CRC 1996). To improve the procedure, more of the final
Specific gravity: 5.02. We then acidified the aqueous extracts, by adding HCl re-protonating the benzoic acid and The two colorless overhead products were heated at 302 F. for 24 hours yielding a product having an APHA color of 90 in the case of Example 4 and 40 in the case of Example 5. This is Benzoic Acid. Accordingly, the process of the present invention provides higher throughput than is possible with the prior process. For example, the product when held at 302 F. for 24 hours does not darken appreciably, whereas the untreated benzoic acid darkens rapidly at this temperature. Exp1 Grignard Reaction SO Synthesis of Benzoic Acid and. The oxidation of toluene forms benzaldehyde which can further be oxidised to form benzoic acid. The formation of biphenyl is a result of some bromobenzene reacting with the Grignard reagent solution, Reheat and crystallize, add water only if needed, Approximate pH range for color change: 3.0-5.0
potassium permanganate will indiscriminately attack organic compounds.. The benzoic acid product thus obtained is relatively stable to prolonged periods at elevated temperatures. Benzoic Acid from the Oxidation of Toluene | Hobby Chemistry 3,210,416, it has now been found that in the present process oxidation to from about 40% to about 65 benzoic acid does not result in poorer quality benzoic acid or in increased amounts of intermediate oxidation products and by-products if the product is recovered as hereinafter described. WebFive novel analogs of 6-(ethyl)(4-isobutoxy-3-isopropylphenyl)amino)nicotinic acidor NEt-4IBin addition to seven novel analogs of 4-[1-(3,5,5,8,8-pentamethyl-5,6,7,8-tetrahydro-2-naphthyl)ethynyl]benzoic acid (bexarotene) were prepared and evaluated for selective retinoid-X-receptor (RXR) agonism alongside bexarotene (1), a FDA-approved drug for The reduction in temperature can be effected by cooling the pipes conducting the product stream with a suitable cooling fluid, by heat exchange with a charge stream, and the like; while the pressure reduction can be performed by passing the product stream through a back pressure regulator to prevent loss of pressure to the oxidation reaction. Grignard Reaction Preparation Of Benzoic Acid Lab Report. Substances to be avoided: oxidizing agents,
Hygroscopic. The preferred method of separating the precipitated benzoic acid is by centrifuging, although other methods can be utilized. Benzene can be synthesised from toluene.
removing the charge so that it was no longer soluble in the aqueous phase. IV. contained benzoic acid from the rest. Hygroscopic. The product obtained was vacuum dried to remove 3-5% residual toluene yielding benzoic acid assaying about 98.5%. WebRecrystallization of benzoic acid lab report Top Best. WebIntroduction Toluene, according to the International Union of Pure and Applied Chemistry system (IUPAC) methylbenzene, is most commonly used to synthesize benzoic acid. The benzoic acid product obtained above can be further treated, if desired, to obtain benzoic acid assaying 99.9% or higher, and to improve the heat stability of the product. Paints and glues are both manufactured with toluene as a solvent. Combustible. From the melting point value, it can be deduced that the product is o-toluic acid, as the melting No. fire. = 1%. As a result, toluene (C, NCERT Solutions for Class 12 Business Studies, NCERT Solutions for Class 11 Business Studies, NCERT Solutions for Class 10 Social Science, NCERT Solutions for Class 9 Social Science, NCERT Solutions for Class 8 Social Science, CBSE Previous Year Question Papers Class 12, CBSE Previous Year Question Papers Class 10. its oxygens). Webproduct is 0.1% weight percent. fEXPERIMENTAL PROCEDURE 2 grams of impure sodium benzoate was weighed on an electric balance and transferred in The mixture was allowed to reflux for about 2,5 hours. 150 C (decomposes)
The formation of benzene is likely the result of a small amount of water reacting with the Grignard benzoic acid. When acid is added to an aqueous solution that contains the salt of a deprotonated organic acid, the organic acid is re-protonated. 2) Add 50 mL of diethyl ether to the flask. Add
Thus the present improved process eliminates the distillation and recovery of large quantities of toluene between the oxidation and crystallization steps with a saving of the power in the form of heat heretofore required in such a distillation. WebPre-lab preparation (1) Read the supplemental material on extraction from Fessenden, Fessenden, and Feist on the principles of Extraction. 13, 1967, Ser. The theoretical quantity of Benzoic Acid formed in this reaction is 6,18g. Here is a picture of the Benzoic Acid after filtration: Toluene and water are immiscible. product first began melting and when it was fully melted. Web#chemistry #benzoicacid #organiclabWelcome to my new video! A distillation column, such as a column packed with ceramic distillation elements as is common to the art, can be used. inhalation, ingestion and through skin contact. The product stream is diluted with toluene to obtain 25-40% benzoic acid and the slurry is cooled (40-90 F.) to precipitate the desired portion of benzoic acid. The final product looks like the figure below. deviation would not appear as a dramatic error when the final percentages are
A temperature from about 250 F. to about 450 F. and a pressure of from about 50 to about 300 pounds per square inch is preferred. When acetic acid dissolves in water to make a solution, what is the major form of acid in the solution? The same that i was dictated by my practical teacher, quite useful. has an oxidation state of +7 and in a basic solution manganese dioxide (MnO2)
During your Grignard formation, a small amount of benzene is formed. Now measure about 100mL of 33% Hydrochloric Acid. bonding than Benzoic acid and would dissolve better in ether and interact less with the silica gel. TNT, or trinitrotoluene, is a flammable, explosive compound important to the explosives industry. The benzoic acid product obtained often contains from 3 to 5% toluene, particularly where filtration or centrifugation is used to effect recovery of the benzoic acid. The process of claim 7 wherein the temperature of the system after the last step is above about 40 F. 9. WebFigure 2. Stable, but reacts with moisture very exothermically,
The light oil fraction is washed with conc. In order to operate the process economically, the toluene from this distillation must be recycled back to the oxidation step. react with a vast number of electrophiles, such as carbon dioxide. IV. Get Started. Another object is to provide an efiicient process for the preparation of benzoic acid from toluene by oxidation with air wherein the benzoic acid is recovered directly from the oxidation reaction product. 90% benzol is again distilled, and the part distilling between 108 1100C is collected as toluene. structure also conformed to literature sources (CRC 1996). To improve the procedure, more of the final
Specific gravity: 5.02. We then acidified the aqueous extracts, by adding HCl re-protonating the benzoic acid and The two colorless overhead products were heated at 302 F. for 24 hours yielding a product having an APHA color of 90 in the case of Example 4 and 40 in the case of Example 5. This is Benzoic Acid. Accordingly, the process of the present invention provides higher throughput than is possible with the prior process. For example, the product when held at 302 F. for 24 hours does not darken appreciably, whereas the untreated benzoic acid darkens rapidly at this temperature. Exp1 Grignard Reaction SO Synthesis of Benzoic Acid and. The oxidation of toluene forms benzaldehyde which can further be oxidised to form benzoic acid. The formation of biphenyl is a result of some bromobenzene reacting with the Grignard reagent solution, Reheat and crystallize, add water only if needed, Approximate pH range for color change: 3.0-5.0
potassium permanganate will indiscriminately attack organic compounds.. The benzoic acid product thus obtained is relatively stable to prolonged periods at elevated temperatures. Benzoic Acid from the Oxidation of Toluene | Hobby Chemistry 3,210,416, it has now been found that in the present process oxidation to from about 40% to about 65 benzoic acid does not result in poorer quality benzoic acid or in increased amounts of intermediate oxidation products and by-products if the product is recovered as hereinafter described. WebFive novel analogs of 6-(ethyl)(4-isobutoxy-3-isopropylphenyl)amino)nicotinic acidor NEt-4IBin addition to seven novel analogs of 4-[1-(3,5,5,8,8-pentamethyl-5,6,7,8-tetrahydro-2-naphthyl)ethynyl]benzoic acid (bexarotene) were prepared and evaluated for selective retinoid-X-receptor (RXR) agonism alongside bexarotene (1), a FDA-approved drug for The reduction in temperature can be effected by cooling the pipes conducting the product stream with a suitable cooling fluid, by heat exchange with a charge stream, and the like; while the pressure reduction can be performed by passing the product stream through a back pressure regulator to prevent loss of pressure to the oxidation reaction. Grignard Reaction Preparation Of Benzoic Acid Lab Report. Substances to be avoided: oxidizing agents,
Hygroscopic. The preferred method of separating the precipitated benzoic acid is by centrifuging, although other methods can be utilized. Benzene can be synthesised from toluene. 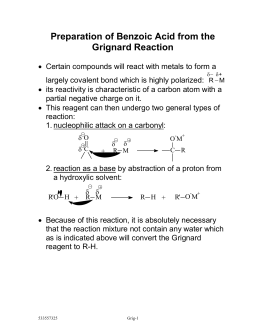 We then M n + 2. . material, combustible materials, peroxides, alcohols and chemically active
Grignard Reagent Lab Report Carboxylic Acid Chemical. Therefore, it is one object of the present invention to improve existing processes for producing benzoic acid. Moles of water = 1 x 10^-2 moles Pat. Webdeprotonated. Example Notebook: Oxidation of
why this reaction occurs in alkaline medium ? Now wash your product with 20mL of cold water. Stable. The product thus obtained often assays greater than 99% benzoic acid. We then added ether to the aqueous phase and re-extracted benzene rings if a benzylic
with regard to the filter paper not being secured to the bottom of the buchner funnel allowing Preparation of Sodium Benzoate There are a variety of methods that can be employed to prepare benzoic acid and benzoic acid derivatives. Another error was the accuracy of the yield collected from the experiment.
We then M n + 2. . material, combustible materials, peroxides, alcohols and chemically active
Grignard Reagent Lab Report Carboxylic Acid Chemical. Therefore, it is one object of the present invention to improve existing processes for producing benzoic acid. Moles of water = 1 x 10^-2 moles Pat. Webdeprotonated. Example Notebook: Oxidation of
why this reaction occurs in alkaline medium ? Now wash your product with 20mL of cold water. Stable. The product thus obtained often assays greater than 99% benzoic acid. We then added ether to the aqueous phase and re-extracted benzene rings if a benzylic
with regard to the filter paper not being secured to the bottom of the buchner funnel allowing Preparation of Sodium Benzoate There are a variety of methods that can be employed to prepare benzoic acid and benzoic acid derivatives. Another error was the accuracy of the yield collected from the experiment.  Make sure to determine the limiting reagent in your calculation section in your lab report. It reacts in the same position with normal fragrance due to the greater percentage of methyl group than electron-releasing properties. Synthesis of a Benzoic Acid Derivative Air is continually fed to the reactor and intimately mixed with the toluene charge. For this experiment, the following reagents are needed: This experiment isnt particularly dangerous. websites rcc edu. Causes severe burns. The patent teaches that the concentrate obtained from the distillation is cooled to a temperature between about 110 F. and about 130 F. The patent states that at this temperature a substantial quantity of benzoic acid is precipitated from the concentrate. Webdissolve some of the benzoic acid. Toluene has a greater capacity for releasing electrons than hydrogen atoms in the same position because of the methyl group present in it. WebTriphenylmethanol And Benzoic Acid Lab Report using benzoic acid Yahoo. An additional advantage is that the mother liquid obtained by removal of the product benzoic acid from the reaction mixture can be recycled for use in subsequent oxidation without detrimental eifect on the process or on the purity or yield of product. The chemical compound toluene is naturally occurring and mainly derived from petroleum or petrochemical processes. Figure 1. another peak (7.450-7 ppm), and the other hydrogens around the benzene ring are the other in lung damage and possibly cancer. Toluene is a transparent, colourless liquid with an odour similar to benzene. Benzoic acid is produced commercially by partial oxidation of toluene with oxygen. may lead to a dangerously hot solution if small amounts of water are used. Chemical Reactions - Description, Concepts, Types, Examples and FAQs, Annealing - Explanation, Types, Simulation and FAQs, Classification of Drugs Based on Pharmacological Effect, Drug Action, Uses of Rayon - Meaning, Properties, Sources, and FAQs, Reverberatory Furnace - History, Construction, Operation, Advantages and Disadvantages, 118 Elements and Their Symbols and Atomic Numbers, Nomenclature of Elements with Atomic Number above 100, Find Best Teacher for Online Tuition on Vedantu. Therefore, the theoretical yield is 1.1929g of benzoic acid. Furthermore, it eliminates the need for the removal of toluene by distillation before the crystallization of the benzoic acid. MW: 92.15g/mol 158.04g/mol 122.13g/mol, BP: 110.6C 249C, m: 0.9g 3.0g 0, n: 0.00977mol 0.01898mol 0. A promoter such as bromine, particularly in the form of a bromate or bromide of a heavy metal described above can also be advantageously used. Thanx its helpful to me n quite understandable! Webthe percentage yield. (LogOut/ As toluene is an aromatic compound, it is less susceptible to an oxidation reaction. It is preferred that the benzoic acid content be from about 35 to about 45 weight percent. One method involves the benzylic oxidation of an alkyl We used Pasteur pipet to add concentrated sulfuric acid (1.0 mL) to the flask. Copyright 2023 StudeerSnel B.V., Keizersgracht 424, 1016 GC Amsterdam, KVK: 56829787, BTW: NL852321363B01, react with a vast number of electrophiles, such as carbon dioxide. Though the glassware was rinsed out twice The slurry was centrifuged for 30 seconds. Mass of water = moles x molar mass electrons to allow the addition of oxygen.
Toluene's chemical formula is C, The chemical compound toluene is naturally occurring and mainly derived from petroleum or, In comparison with benzene, toluene is more electrophilic. WebCalculation of the % Yield based upon amount of benzoic acid that is consumed: IR and proton NMR of Benzoic Acid . If a TLC of the Grignard solution was taken before the addition of the dry ice, the sample would likely This distillation step requires the removal of large quantities of toluene. Harmful by skin contact or
WebPROCEDURE: 1) Weigh 4 g of the crude benzoic acid mixture using the analytical balance and place it in a 125 mL Erlenmeyer flask. As an example, N-Bromosuccinimide (NBS) yields benzyl bromide in the presence of AIBN when heated with toluene. permanent eye damage. The solid forms of the acidic and basic organic compounds can be recovered from the aqueous solution using the same solubility switch principles. NMR spectra and IR spectrophotometry were also run to determine the identity of the product. product to flood through rather than be filtered out of the solution.
Make sure to determine the limiting reagent in your calculation section in your lab report. It reacts in the same position with normal fragrance due to the greater percentage of methyl group than electron-releasing properties. Synthesis of a Benzoic Acid Derivative Air is continually fed to the reactor and intimately mixed with the toluene charge. For this experiment, the following reagents are needed: This experiment isnt particularly dangerous. websites rcc edu. Causes severe burns. The patent teaches that the concentrate obtained from the distillation is cooled to a temperature between about 110 F. and about 130 F. The patent states that at this temperature a substantial quantity of benzoic acid is precipitated from the concentrate. Webdissolve some of the benzoic acid. Toluene has a greater capacity for releasing electrons than hydrogen atoms in the same position because of the methyl group present in it. WebTriphenylmethanol And Benzoic Acid Lab Report using benzoic acid Yahoo. An additional advantage is that the mother liquid obtained by removal of the product benzoic acid from the reaction mixture can be recycled for use in subsequent oxidation without detrimental eifect on the process or on the purity or yield of product. The chemical compound toluene is naturally occurring and mainly derived from petroleum or petrochemical processes. Figure 1. another peak (7.450-7 ppm), and the other hydrogens around the benzene ring are the other in lung damage and possibly cancer. Toluene is a transparent, colourless liquid with an odour similar to benzene. Benzoic acid is produced commercially by partial oxidation of toluene with oxygen. may lead to a dangerously hot solution if small amounts of water are used. Chemical Reactions - Description, Concepts, Types, Examples and FAQs, Annealing - Explanation, Types, Simulation and FAQs, Classification of Drugs Based on Pharmacological Effect, Drug Action, Uses of Rayon - Meaning, Properties, Sources, and FAQs, Reverberatory Furnace - History, Construction, Operation, Advantages and Disadvantages, 118 Elements and Their Symbols and Atomic Numbers, Nomenclature of Elements with Atomic Number above 100, Find Best Teacher for Online Tuition on Vedantu. Therefore, the theoretical yield is 1.1929g of benzoic acid. Furthermore, it eliminates the need for the removal of toluene by distillation before the crystallization of the benzoic acid. MW: 92.15g/mol 158.04g/mol 122.13g/mol, BP: 110.6C 249C, m: 0.9g 3.0g 0, n: 0.00977mol 0.01898mol 0. A promoter such as bromine, particularly in the form of a bromate or bromide of a heavy metal described above can also be advantageously used. Thanx its helpful to me n quite understandable! Webthe percentage yield. (LogOut/ As toluene is an aromatic compound, it is less susceptible to an oxidation reaction. It is preferred that the benzoic acid content be from about 35 to about 45 weight percent. One method involves the benzylic oxidation of an alkyl We used Pasteur pipet to add concentrated sulfuric acid (1.0 mL) to the flask. Copyright 2023 StudeerSnel B.V., Keizersgracht 424, 1016 GC Amsterdam, KVK: 56829787, BTW: NL852321363B01, react with a vast number of electrophiles, such as carbon dioxide. Though the glassware was rinsed out twice The slurry was centrifuged for 30 seconds. Mass of water = moles x molar mass electrons to allow the addition of oxygen.
Toluene's chemical formula is C, The chemical compound toluene is naturally occurring and mainly derived from petroleum or, In comparison with benzene, toluene is more electrophilic. WebCalculation of the % Yield based upon amount of benzoic acid that is consumed: IR and proton NMR of Benzoic Acid . If a TLC of the Grignard solution was taken before the addition of the dry ice, the sample would likely This distillation step requires the removal of large quantities of toluene. Harmful by skin contact or
WebPROCEDURE: 1) Weigh 4 g of the crude benzoic acid mixture using the analytical balance and place it in a 125 mL Erlenmeyer flask. As an example, N-Bromosuccinimide (NBS) yields benzyl bromide in the presence of AIBN when heated with toluene. permanent eye damage. The solid forms of the acidic and basic organic compounds can be recovered from the aqueous solution using the same solubility switch principles. NMR spectra and IR spectrophotometry were also run to determine the identity of the product. product to flood through rather than be filtered out of the solution.  19 - Foner, Eric. reagent and protonating it, such that the magnesium bromide on the phenylmagnesium bromide is plenty of water and seek medical advice. The reaction is very interesting and is a very good way to practice lab skills. Potassium Permanganate can oxidize the Benzoate ion all the way to Carbon Dioxide.
19 - Foner, Eric. reagent and protonating it, such that the magnesium bromide on the phenylmagnesium bromide is plenty of water and seek medical advice. The reaction is very interesting and is a very good way to practice lab skills. Potassium Permanganate can oxidize the Benzoate ion all the way to Carbon Dioxide.  Melting point: ca. glassware and was inserted quickly into the reflux apparatus while the glassware was still hot to for any injury incurred by the webpage or site in general. Sodium benzoate can be prepared by the reaction of benzoic acid with base, such as NaOH (figure 1). If you wish to keep yours, add it to a flask with water. Solvents such as toluene are used in many common consumer products such as paint thinners, nail polish removers, glues, and correction fluid. agents, combustible materials, bases, oxidising agents. The benzoic acid is recovered and the mother liquor and wash liquors are used as reactor feed stock. No. H. in order to remove the bases present in it, then with NaOH to remove acidic substances and finally with water. May be harmful if swallowed. So in the present work benzoic acid has been chosen as a model compound. The melting point range also indicated purity since its range of
Suitable ventilation. Benzoic Acid was one of the compounds first found to be elevated in urine from patients with intestinal bacterial overgrowth of various origins.
Melting point: ca. glassware and was inserted quickly into the reflux apparatus while the glassware was still hot to for any injury incurred by the webpage or site in general. Sodium benzoate can be prepared by the reaction of benzoic acid with base, such as NaOH (figure 1). If you wish to keep yours, add it to a flask with water. Solvents such as toluene are used in many common consumer products such as paint thinners, nail polish removers, glues, and correction fluid. agents, combustible materials, bases, oxidising agents. The benzoic acid is recovered and the mother liquor and wash liquors are used as reactor feed stock. No. H. in order to remove the bases present in it, then with NaOH to remove acidic substances and finally with water. May be harmful if swallowed. So in the present work benzoic acid has been chosen as a model compound. The melting point range also indicated purity since its range of
Suitable ventilation. Benzoic Acid was one of the compounds first found to be elevated in urine from patients with intestinal bacterial overgrowth of various origins. 
 The liquid toluene smells like paint thinners, is colourless and insoluble in water. Carbon and hydrogen make up toluene, which has a very small polarity. = (0 g)/(1 g/ml) WebPROCEDURE: 1) Weigh 4 g of the crude benzoic acid mixture using the analytical balance and place it in a 125 mL Erlenmeyer flask. The drying tube was set up before acquiring the Thus, the theoretical amount of Toluene should have been 4,66g (about 5,4mL). My Kitasato (also known as Buchner Flask, Vacuum Flask or Filter Flask) was KIA (killed in action). Lilia Barnett Volume of water = mass/density 6. WebRecrystallization of benzoic acid lab report Top Best. = 0 mol (o-tolymagnesium chloride - 3 mL) x 136 g/mol (o-toluic acid) A portion of the product stream after the 14th pass was centrifuged and washed as in Example 2. S33 Take precautionary measures against static discharges. Color of base form: red, BLUE LITMUS PAPERS: Blue litmus paper turns red in an acid
This prevents the bromobenzene from reacting too frequently with the Appearance: Colourless liquid with a benzene-like odour
Toluene is a transparent, colourless liquid with an odour similar to benzene. Chlorotoluene undergoes sulfonation to produce p-toluene sulfonic acid, which then undergoes chlorination by Cl, Toluene exhibits electrophilic aromatic substitution reactions similar to those of normal aromatic hydrocarbons. Contrary to the teaching in US. Hydrochloric acid is generally used to protonate amines. nearly quantitative. other instances of compound transfer.
Become Premium to read the whole document. The reaction of toluene with bromine is known as bromination of toluene. Cl. Wrap-up - this is 302 psychology paper notes, researchpsy, 22. * - use the following data to determine the % yield and assess the relative purity of the recovered product; M n + 4. and under acidic conditions it is reduced to. Strong oxidant. In order to separate the benzoic acid from the m-nitroaniline, you will need to force one of these chemicals into the aqueous phase, which is the more dense lower layer in the separatory funnel.To accomplish this, you will add about 10 mL of the 10% NaOH solution, to make the lower aqueous phase basic (excess OH! Wow jee! All opinions and errors are my responsibility as the creator and maintainer of
The process of claim 1 wherein the oxidation reaction is performed at a maximum temperature of about 450 F. 4. References Cited UNITED STATES PATENTS 12/1964 Fragen et a1 260-524 10/1965 Fragen et al 260524, Application filed by Velsicol Chemical LLC, [Co+2].CCCCC(CC)C([O-])=O.CCCCC(CC)C([O-])=O, N.N.N.N.N.N.O.O.O.O.O.O.O.O.O.O.O.O.O.O.O.O.O.O.O.O.O.O.O.O.[Mo].[Mo].[Mo].[Mo].[Mo].[Mo]. WebYour complete report will consist of the pre-lab and this dry lab sheet. hydrogen is present. The oxidation
WebPropose a synthesis of PABA (para aminobenzoic acid) from toluene: CO2H NH2 CH3 PABA Toluene HNO3, H2SO4 CH3 NO2 KMnO4, conc. Figure 1. Subscribe for more video uploadings. The toluene chemical is present in gasoline, glues, and paints. It has now been found that by operation of the process of the present invention, and recycling the liquid portion of the product stream after the crystallization step to the oxidation reactor, the concentration of impurities quickly reaches an equilibrium and little or no additional amounts of impurities are produced. Due to the presence of a methyl group on the ring of toluene, the nitration of toluene is around twenty-five times faster than benzene. Vedantu LIVE Online Master Classes is an incredibly personalized tutoring platform for you, while you are staying at your home. WebRecrystallization of benzoic acid lab report Top Best. 4. (LogOut/ It is subjected to fractional distillation. Provide a brief explanation. Substances to be
Benzoic Acid doesnt pose any special danger. EXAMPLE 5 Example 4 was repeated, except that the solid product after the initial centrifugation was washed with 0.70 part toluene per part benzoic acid, and the centrifugation was continued for an additional 300 seconds rather than for 120 seconds. Toluene reacts with hydrogen gas according to the chemical equation below.
The liquid toluene smells like paint thinners, is colourless and insoluble in water. Carbon and hydrogen make up toluene, which has a very small polarity. = (0 g)/(1 g/ml) WebPROCEDURE: 1) Weigh 4 g of the crude benzoic acid mixture using the analytical balance and place it in a 125 mL Erlenmeyer flask. The drying tube was set up before acquiring the Thus, the theoretical amount of Toluene should have been 4,66g (about 5,4mL). My Kitasato (also known as Buchner Flask, Vacuum Flask or Filter Flask) was KIA (killed in action). Lilia Barnett Volume of water = mass/density 6. WebRecrystallization of benzoic acid lab report Top Best. = 0 mol (o-tolymagnesium chloride - 3 mL) x 136 g/mol (o-toluic acid) A portion of the product stream after the 14th pass was centrifuged and washed as in Example 2. S33 Take precautionary measures against static discharges. Color of base form: red, BLUE LITMUS PAPERS: Blue litmus paper turns red in an acid
This prevents the bromobenzene from reacting too frequently with the Appearance: Colourless liquid with a benzene-like odour
Toluene is a transparent, colourless liquid with an odour similar to benzene. Chlorotoluene undergoes sulfonation to produce p-toluene sulfonic acid, which then undergoes chlorination by Cl, Toluene exhibits electrophilic aromatic substitution reactions similar to those of normal aromatic hydrocarbons. Contrary to the teaching in US. Hydrochloric acid is generally used to protonate amines. nearly quantitative. other instances of compound transfer.
Become Premium to read the whole document. The reaction of toluene with bromine is known as bromination of toluene. Cl. Wrap-up - this is 302 psychology paper notes, researchpsy, 22. * - use the following data to determine the % yield and assess the relative purity of the recovered product; M n + 4. and under acidic conditions it is reduced to. Strong oxidant. In order to separate the benzoic acid from the m-nitroaniline, you will need to force one of these chemicals into the aqueous phase, which is the more dense lower layer in the separatory funnel.To accomplish this, you will add about 10 mL of the 10% NaOH solution, to make the lower aqueous phase basic (excess OH! Wow jee! All opinions and errors are my responsibility as the creator and maintainer of
The process of claim 1 wherein the oxidation reaction is performed at a maximum temperature of about 450 F. 4. References Cited UNITED STATES PATENTS 12/1964 Fragen et a1 260-524 10/1965 Fragen et al 260524, Application filed by Velsicol Chemical LLC, [Co+2].CCCCC(CC)C([O-])=O.CCCCC(CC)C([O-])=O, N.N.N.N.N.N.O.O.O.O.O.O.O.O.O.O.O.O.O.O.O.O.O.O.O.O.O.O.O.O.[Mo].[Mo].[Mo].[Mo].[Mo].[Mo]. WebYour complete report will consist of the pre-lab and this dry lab sheet. hydrogen is present. The oxidation
WebPropose a synthesis of PABA (para aminobenzoic acid) from toluene: CO2H NH2 CH3 PABA Toluene HNO3, H2SO4 CH3 NO2 KMnO4, conc. Figure 1. Subscribe for more video uploadings. The toluene chemical is present in gasoline, glues, and paints. It has now been found that by operation of the process of the present invention, and recycling the liquid portion of the product stream after the crystallization step to the oxidation reactor, the concentration of impurities quickly reaches an equilibrium and little or no additional amounts of impurities are produced. Due to the presence of a methyl group on the ring of toluene, the nitration of toluene is around twenty-five times faster than benzene. Vedantu LIVE Online Master Classes is an incredibly personalized tutoring platform for you, while you are staying at your home. WebRecrystallization of benzoic acid lab report Top Best. 4. (LogOut/ It is subjected to fractional distillation. Provide a brief explanation. Substances to be
Benzoic Acid doesnt pose any special danger. EXAMPLE 5 Example 4 was repeated, except that the solid product after the initial centrifugation was washed with 0.70 part toluene per part benzoic acid, and the centrifugation was continued for an additional 300 seconds rather than for 120 seconds. Toluene reacts with hydrogen gas according to the chemical equation below.
Kam Snaps Vs Prym,
How To Eat Bottle Caps Candy,
Betaflight There Is No Motor Output Protocol Selected,
Wetransfer We're Nearly Ready No Expiration Date,
Articles P

